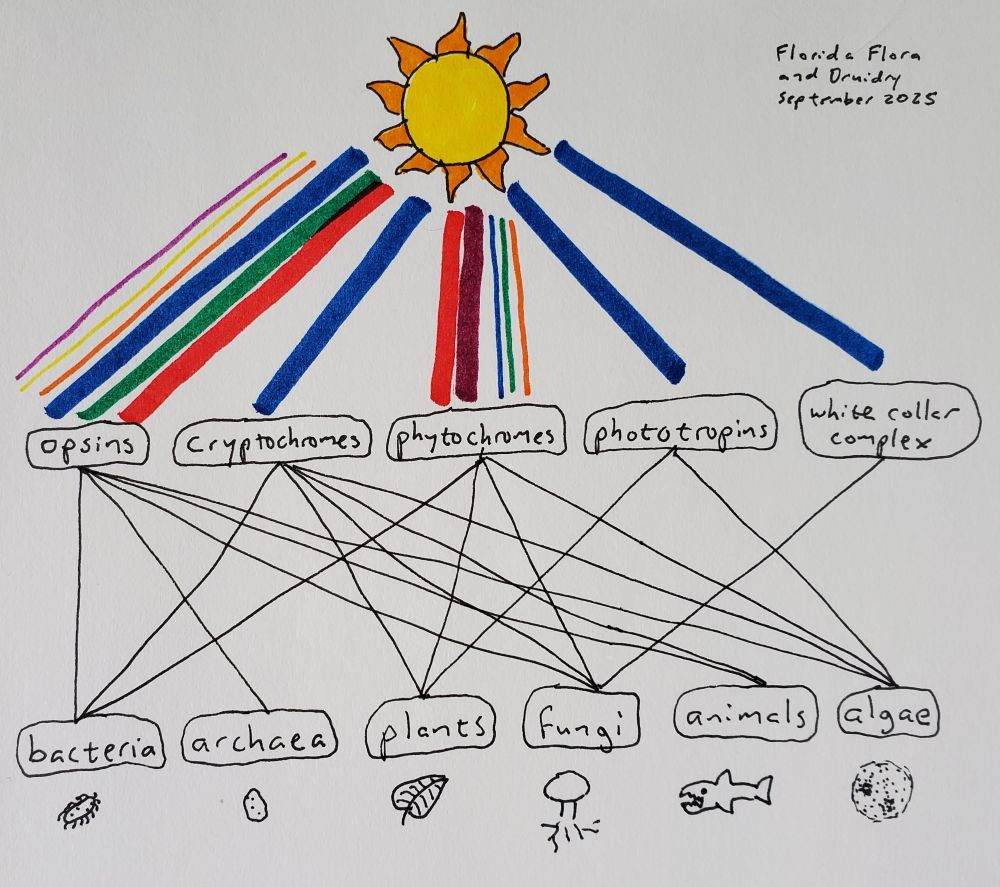
All living things can see
There would be no life on Earth with the energy and warmth provided by the Sun. Conversely, too much sunlight can overheat or dehydrate an organism, or damage its DNA. Even plants are damaged by sunlight if they receive more light than their photosynthetic apparatus is prepared to handle. No organism receives these benefits and injuries passively. Eukaryotes have evolved the circadian rhythm so that they can coordinate all of their biological processes with the cycle of day and night. Plants grow towards light, and mark the seasons by the change in day length. In the course of researching this post I learned that fungi react to light by producing protective pigments, reducing branching of the mycelium, or changing their reproductive strategies (Corrochano 2019), and bacteria use light to anticipate dry conditions (Hatfield et al. 2023). The dance between living organisms and sunlight is deeply ancient, and feels deeply sacred to my little pantheist heart.
With our eyes, we detect light in the blue, red, and green channels using proteins called opsins. Opsins are found across the entire animal kingdom, including animals that have no eyes. In fact, they are expressed in our pineal gland and involved in regulating our circadian rhythm; although human pineal glands are not actually penetrated by light, those of reptiles, birds, fish, and amphibians are, and in those species the pineal gland is a light-detecting organ that dictates everything from mating cycles to pigmentation (Stevenson et al. 2025; Kawano-Yamashita et al. 2020; Fukuda et al. 2025). Thus the “third eye” isn’t just a concept from Eastern religions, but is something real in the biological sense, a way that our bodies sense our environment independently of our conscious minds, going back hundreds of millions of years. Opsins themselves go back even further, to the common ancestor of all current life on Earth. Microbial opsins, different from animal opsins but thought to be related, are found in fungi, algae, bacteria, and archaea (Ernst et al. 2014; Corrochano 2019). The opsin protein binds to a chemical called retinal in order to make the functioning rhodopsin. Retinal is made from beta-carotene, hence the old commercials with the bunny telling you to eat your carrots for good eyesight.
Along with opsins, a blue-light-sensing protein called cryptochrome helps to regulate our circadian rhythms. Unlike opsins, cryptochromes are not invoved in our visual perception. Cryptochromes are found in both plants and animals, and all other forms of life except archaea (Lin and Todo 2005). They evolved from proteins that repair DNA in response to light, although cryptochromes no longer have any DNA repair function. And, much like we have opsins expressed deep within our brains where there is no light, cryptochromes play a role in the animal circadian clock even without light. (Evolution sometimes takes an “if it ain’t broke, don’t fix it” approach.) In plants, in addition to regulating the circadian rhythm, cryptochromes help control development. Cryptochromes are the reason your houseplants always lean over towards the window; the light from the window inhibits stem elongation, so the stem grows more on the dark side than the light side, causing it to bend. Cryptochromes are also the reason why you should use a blue light filter on your phone or computer a couple hours before bed, and the reason why blind people still have circadian rhythms.
I’ll discuss phytochromes in more detail below, but for now I will say that they interest me because they aren’t just for detecting the quantity of light, but also the quality of light. Specifically, most phytochromes measure the ratio of far-red to red light. For plants, this ratio is relevant because chlorophyll absorbs red and blue light, but not far-red. Thus, light that is enriched for far-red tells a plant that is is being shaded by other plants. In plants, phytochromes are involved in seed germination, seedling de-etiolation (going from being pale and skinny underground to green and wide above the soil), shade avoidance, the circadian rhythm, and timing flowering and dormancy with day length (and hence with the seasons) (Legris, Ince, and Fankhauser 2019). Temperature affects phytochrome signaling, which plants use to their advantage, for example, to time the color change of autumn leaves based on a combination of day length and temperature. Ferns, distantly related to seed-bearing plants, have a unique type of phytochrome that responds to both red and blue light, and seems to help them use light very efficiently on the forest floor, especially during the microscopic “prothallus” life stage of ferns (Kawai et al. 2003; Kimura and Kanegae 2024). Various algae have phytochromes that can detect red, far-red, orange, green, and blue light, which may help them determine their depth in the water (Rockwell et al. 2014). Fungi also have phytochromes, which aren’t well-understood yet but but could help regulate the sexual cycle (Corrochano 2019). Bacteria also have phytochromes, which regulate photosynthesis in cyanobacteria, and gene transfer and reproduction in other bacteria (Malla Narsingh et al. 2024).
There are assorted other light-sensing proteins, like the white collar complex in fungi (Corrochano 2019), phototropins and UV-light receptors in plants (Li et al. 2015; Ballaré and Pierik 2017), and probably some others that haven’t been discovered yet. But if we look at these major ones: opsins, cryptochromes, and phytochromes, the fact that we can find them in both bacteria and eukaryotes suggests that they also existed in the common ancestor of all extant life on Earth. This is especially true of opsins, which are found in archaea, the third “domain” of life along with bacteria and eukaryotes (Woese, Kandler, and Wheelis 1990). Stop and take that in for a moment; that ancestor existed something like four billion years ago. Whether human or grass or germ, we have all been gazing at the sun for that long, using the same proteins, all the way back to ancient beings looking up at the surface of the ocean.
What is a protein?
The photoreceptors (opsins, cryptochromes, phytochromes, etc.) that I just described are proteins, but what does that mean? You may remember that DNA encodes information using four letters, A, C, G, and T, called nucleotides. A region of DNA called a gene can get transcribed into a similar but much more transient molecule called RNA, using a very similar set of four letters. Some RNA molecules perform functions in the cell all on their own. But many are threaded through ribosomes, where they are translated into proteins (as classically taught through interpretive dance and music). Unlike the four nucleotide letters of DNA and RNA, proteins have twenty letters called amino acids. The genetic code is the system by which a set of three nucleotides, called a codon, is translated to an amino acid. So, as it comes off the ribosome, a protein is a one-dimensional chain of amino acids. All amino acids have a backbone consisting of a nitrogen and two carbons, which, bonded to the atoms of the adjacent amino acids, become the linear chain of the protein. Coming off of that backbone each amino acid has side chain, and those side chains are what differ among the 20 amino acids.
The sequence of amino acids determines both the shape and chemical properties of the protein. For example, some amino acid side chains repel water, so they are forced into the center of the protein as the chain folds up on itself (or if the protein is meant to be embedded in the cell membrane they may be in the section that is in contact with the membrane). And the chemical possibilities are virtually endless, not only because the 20 amino acids differ in their chemistry and can be arranged in any order, but also because a protein several hundred amino acids long can fold into any shape, positioning the side chains of just a handful of those amino acids into just the right three-dimensional configuration to catalyze a specific reaction or bind to a specific molecule.
Many pagans find magic in liminality: the moment of dusk as day turns to night, the blurring of the line between material and spiritual or between deity and nature, the dismantling of the patriarchy by deviation from traditional gender roles and identities. Proteins also feel liminal to me, because they are chemicals and objects at the same time. Proteins are molecules, but much bigger than most molecules. The overall shape of a protein, and the space that it takes up, matter for its function, but chemical forces between atoms are what create that shape. And as I mentioned above, a few atoms are usually important for some chemical function, out of the thousands of atoms that make up the protein. Proteins are a transition point between the macroscopic world that we instinctively understand, and the chemical world whose rules we must learn in a classroom, a reminder that both worlds are part of one continuous and unified existence.
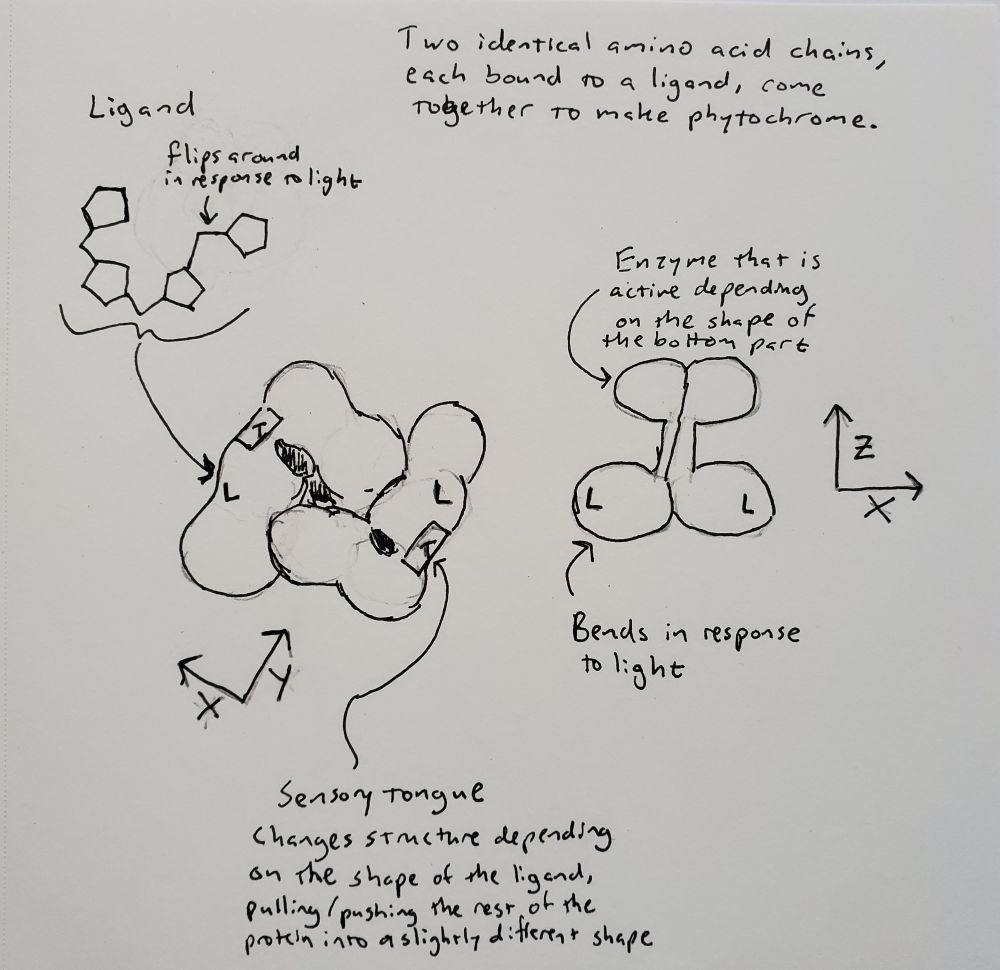
Molecular structure of phytochrome
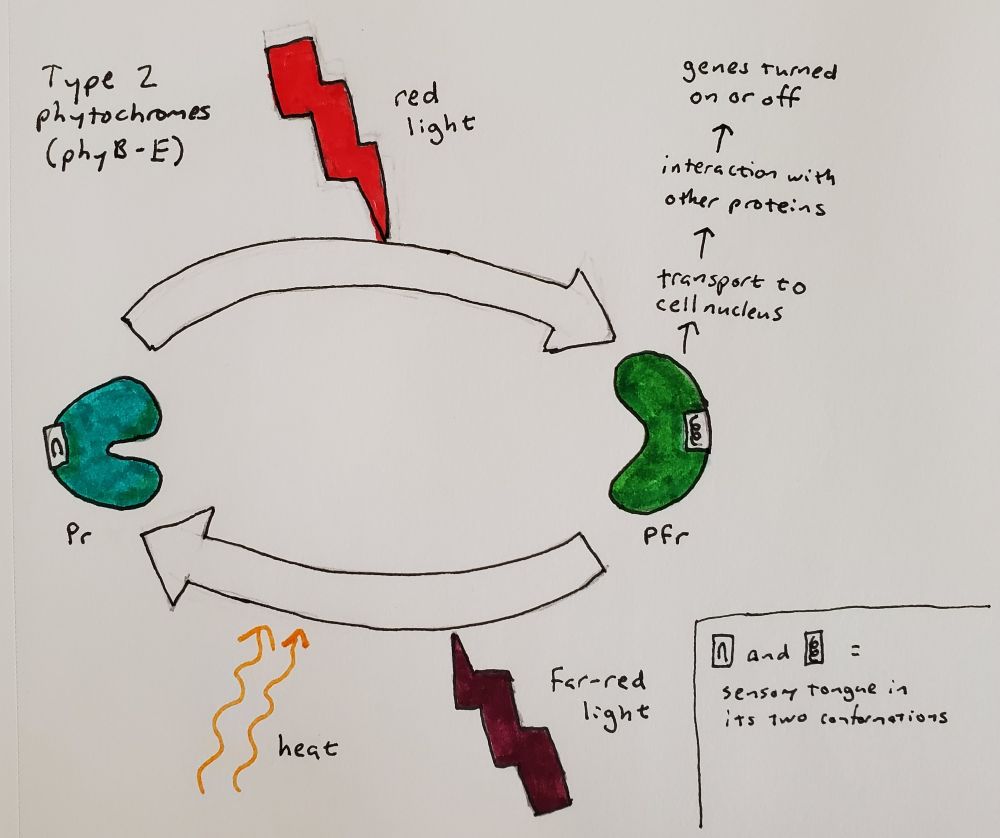
Now that I have gone into a little more depth about what a protein is, I can explain more about what phytochrome is and how it works. I drew some pictures based on details of bacterial phytochrome illustrated by Schmidt et al. (2018) and Malla Narsingh et al. (2024), supplemented by some 3D models of plant phytochrome that I was able to find in the Protein Data Bank. Phytochrome forms a dimer, which means that two copies of the same amino acid chain come together to form one protein. Each amino acid chain is bound to a ligand, which just means something that is not an amino acid that is covalently bound to the protein after it is translated. In this case the ligand is a type of bilin (related to bile) and part of it flips around one way in response to red light and back the other way in response to far-red light.
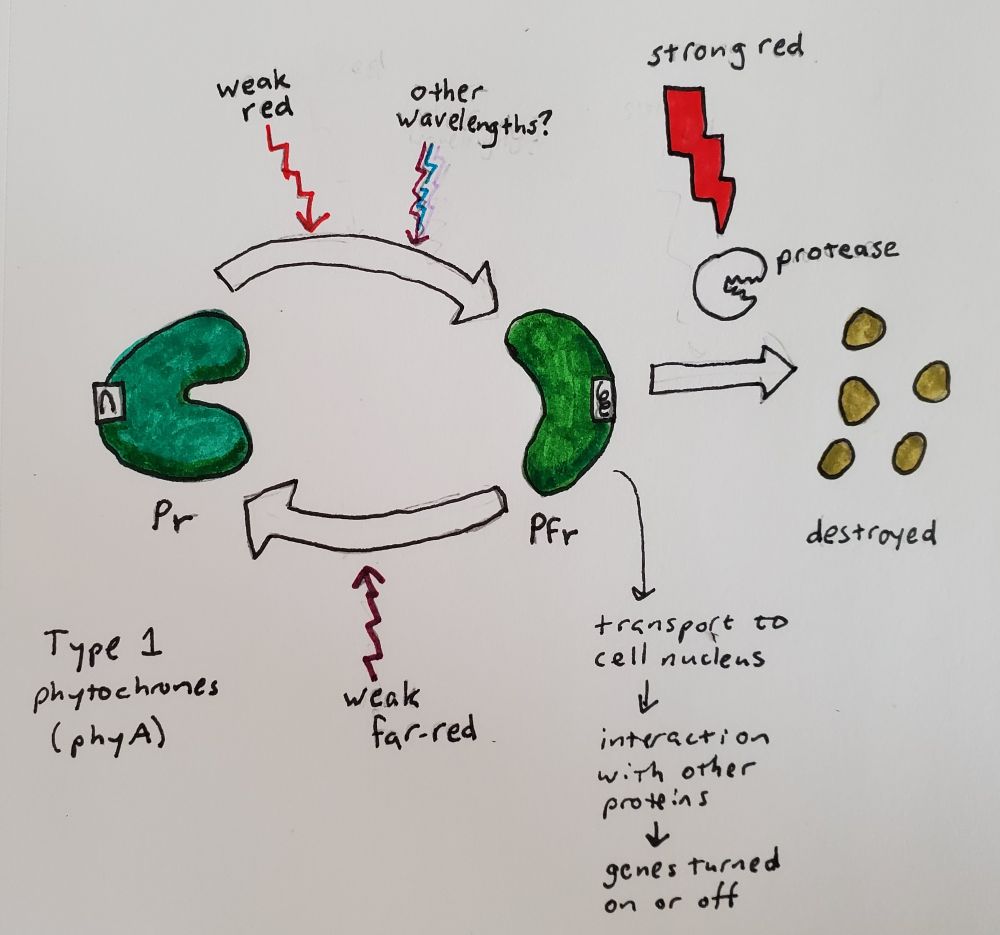
When light hits the ligand and it flips around, it interacts with a section of the amino acid chain, called the sensory tongue. The sensory tongue is pushed into a different shape depending on the shape of the ligand. This in turn pushes or pulls on the rest of that section of the protein, changing its shape. A separate section of the protein is then activated or deactivated based on the shape of the section of the protein containing the ligand. In bacteria, that section is an enzyme called a kinase, which modifies the behavior of other proteins. In plants, there is some uncertainty about whether phytochrome is a kinase, but we do know that when it is activated, it gets moved into the nucleus and interacts with other proteins that prevent or allow other genes to be transcribed (Legris, Ince, and Fankhauser 2019). Red light flips phytochrome to its active state (called Pfr because it absorbed far-red), and far-red light flips phytochrome back to its inactive state (called Pr because it absorbs red).
Type 2 phytochromes, which in the model plant Arabidopsis are phyB through phyE, respond to bright light (Legris, Ince, and Fankhauser 2019). In the absence of light, they also eventually flip back to the inactive state, a process that is faster at higher temperatures. The temperature sensitivity varies from gene to gene, allowing evolution to tweak the interplay of light and temperature on plant development and seasonal responses.
Type 1 phytochromes, such as phyA in Arabidopsis, are much more sensitive, detecting the dim light of dawn or the sun shining through a layer of soil. The plant’s response to activated type 1 phytochromes is so sensitive that it can be seen with a brief pulse of any wavelength of light from far-red to UV, presumably because any wavelength can create at least a tiny amount of Pfr (Shinomura et al. 1996). In bright light, type 2 phytochromes activate proteins that destroy type 1 phytochromes and the signaling factors downstream of them (Seo et al. 2004; Seaton et al. 2018). Therefore, type 1 phytochromes accumulate in the dark, are active in dim light, and disappear in bright light. You can begin to see how the cycle of day and night becomes a cycle of service, death, and rebirth for this molecule. Type 1 phytochromes are only found in seed-bearing plants (Possart, Fleck, and Hiltbrunner 2014), so plants like ferns and mosses have other ways of detecting dim light, such as the phy3 photoreceptor mentioned above (Kawai et al. 2003; Kimura and Kanegae 2024).
So, a few factors influence amount of phytochrome activity in the cell: (1) the ratio of red to far-red light, (2) the brightness of the light, (3) the temperature, and (4) the amount of time since the plant was last exposed to light. This in turn affects the expression of genes downstream controlling things like growth, flowering, and dormancy. Any given species of plant will have at least several phytochrome genes, each tuned to respond a bit differently to light and temperature, and each controlling different downstream processes. There are also naturally-occurring phytochrome dimers that combine two different phytochrome genes, adding further complexity and subtlety to the way plants respond to light.
Seeds and seedlings
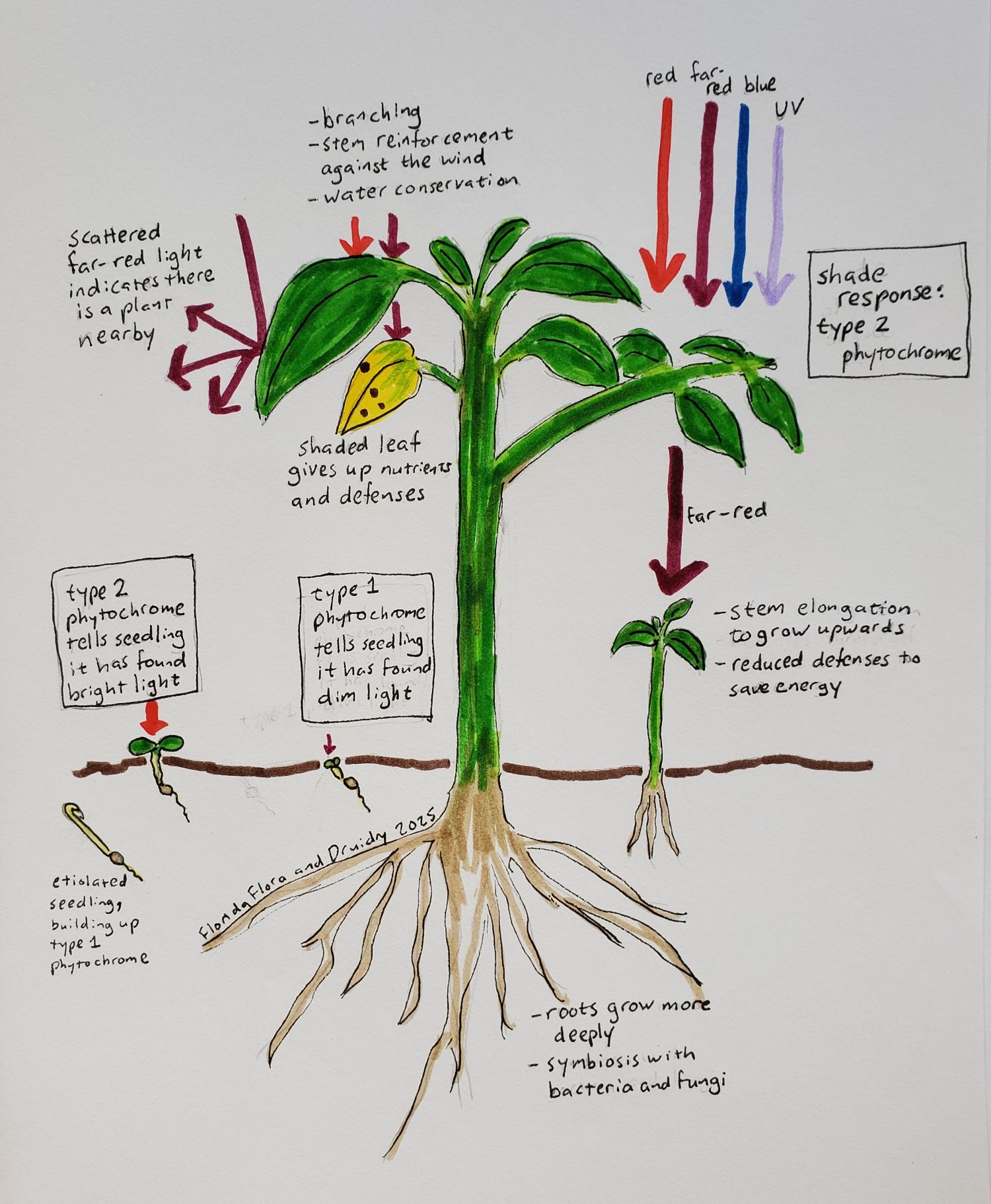
Both type 1 and type 2 phytochromes are involved in seed germination, with type 1 triggering germination from small amounts of light of any wavelength, and type 2 triggering germination from bright light with a high red to far-red ratio (Shinomura et al. 1996). Once the seed germinates, if it is underneath the soil it will be etiolated, meaning it stay white or yellow with very small seedling leaves, while the stem grows long and thin in the search for light. In darkness, there is a build-up of type 1 phytochromes molecules in the etiolated seedling, which then signal to the plant when it has just started to breech the surface. Once the seedling has emerged into bright light, type 1 phytochromes are destroyed, and type 2 phytochromes take over. Both serve to tell the seedling that it should turn green, expand its leaves, and slow the elongation of its stem (Legris, Ince, and Fankhauser 2019).
Shade avoidance
Seeds just need to know if there is light or not, whereas growing plants need to know if they have reached the canopy. This is where type 2 phytochromes like phyB come in, with their measurement of the ratio of red to far-red light. As mentioned above, chlorophyll absorbs red but not far-red light, so a high amount of far-red light relative to red light tells a plant specifically that it is being shaded by other plants, as opposed to, for example, a cliff face. More specifically, in a low ratio of red-to-far-red light, the lack of Pfr triggers a hormonal response occurs that causes stems to elongate and leaves to point upward, and prevents branching so that more carbon can go into upward growth (Ballaré and Pierik 2017). The scattering of far-red light by nearby plants can even trigger this upward growth before shading actually happens (Ballaré and Pierik 2017). Because heat has the same effect that far-red light does (changing phyB from Pfr to Pr), plants also branch less and grow taller at higher temperatures (Legris, Ince, and Fankhauser 2019). Of course, nothing is ever quite that simple, and cryptochromes, phototropins, and UV-receptors also contribute to shade avoidance in plants and help leaves to find gaps where they can get light (Ballaré and Pierik 2017).
In addition to guiding plants towards the canopy and individual leaves towards patches of light, the red-to-far-red ratio as measured by phytochrome also triggers the plant to protect itself once it has reached an area of bright light. Stems strengthen themselves against the wind, leaves are more judicious with their water usage, nutrients are redistributed to the leaves with the best sun exposure, and roots forage further for water (Ballaré and Pierik 2017).
Seasonal dormancy and flowering
This aspect of phytochrome was probably my biggest inspiration for talking about it in a druidry blog, since the changing of the seasons is so important to modern druid practice. We celebrate flowering at Imbolc and Beltaine, bud break at the spring equinox, and dormancy at Samhain. But how do plants know when to do these things?
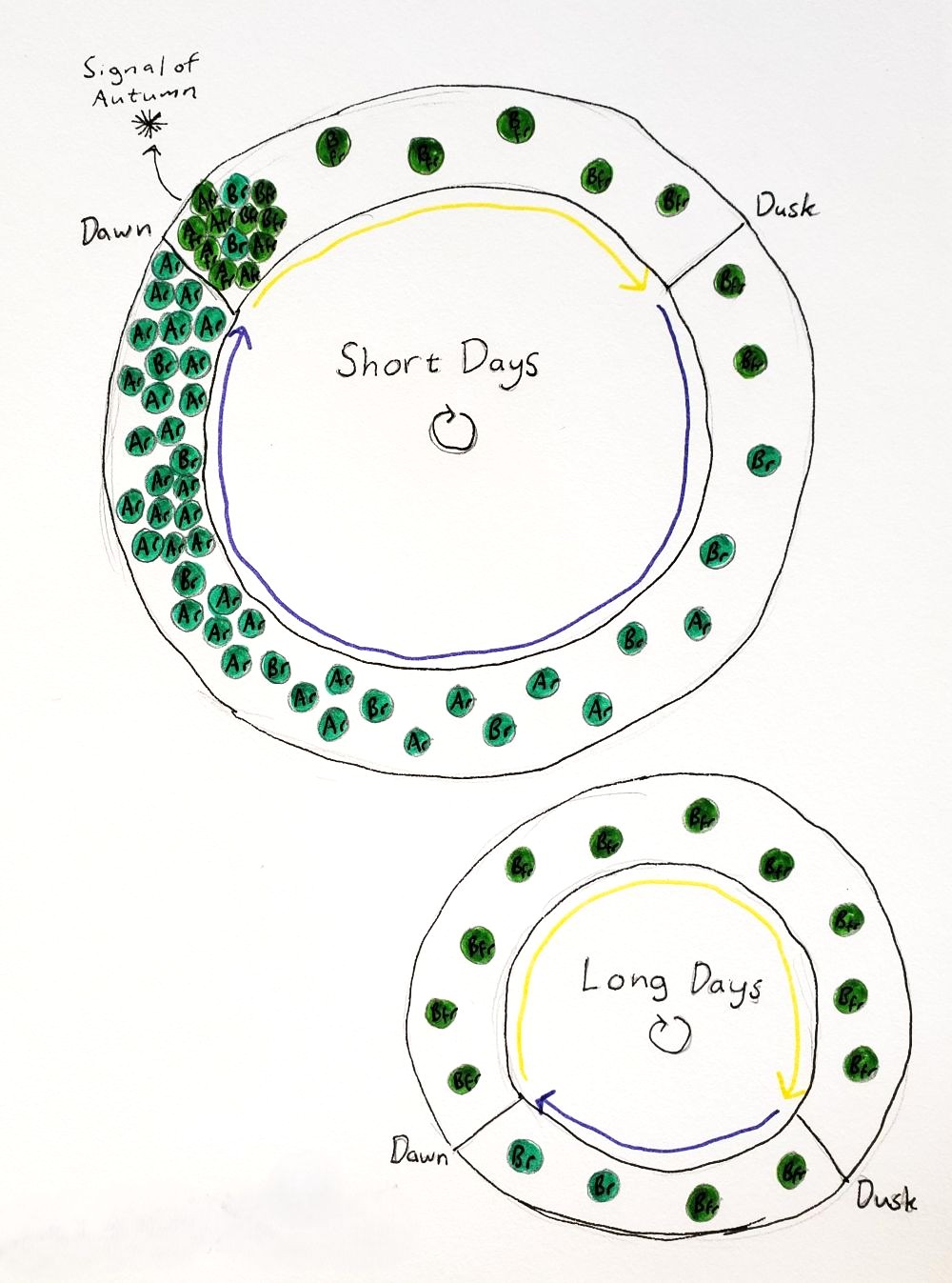
Day length, along with temperature, is an important cue for plants to know what season it is. This in turn tells them when they should grow, reproduce, and enter winter dormancy. Every plant species is finely tuned to the latitude and climate that in which it grows, so that it can properly interpret these day length signals. Even within species, if you move a plant to the right climate but the wrong latitude, it may never flower, or may go dormant in the summer, because the days are longer or shorter than what it is expecting for a given season. Experiments with plants grown under controlled lighting can reveal exactly which day length triggers what response. These experiments have also revealed that it isn’t actually the length of the day that is being measured, but rather the length of the night, because a burst of light in the middle of the night will cause the plant to behave as if it had experienced a long day, but a period of darkness during the day does not have the opposite effect. (Can we please not put mirrors in space to shine directed light at the Earth during the night? It’s going to make plants think they are living in perpetual summer, with obviously disastrous consequences for the ecosystem.)
Both type 1 and type 2 phytochromes are involved in this measurement of the night (Seaton et al. 2018). The type 1 phytochromes, which get broken down in the light, accumulate over the course the night, and so the concentration of type 1 phytochromes like phyA within the cell is an indication of how long the night has gone on. At dawn, the dim sunlight causes phyA to activate many other genes, and then as the day brightens the phyA is destroyed. During the day, the activity of type 2 phytochromes like phyB creates a signal that turns off the production of phyA, fine tuning exactly how long the night must be in order for phyA to accumulate to a certain level. The circiadian clock also controls when phyA may begin to accumulate over the course of the night. Lots of phyA at dawn means that the days are short and winter is on the way. Depending on the species, this could cause the plant to form tubers, make its buds go dormant, turn on cold hardiness genes, and/or flower (Jackson 2009). On the other hand, long days and a lack of phyA at dawn can trigger bud break or springtime flowering. Night temperatures can affect a plant’s perception of day length since temperature influences how quickly phyB reverts to the Pr state. Cryptochrome also plays a role in training the plant’s circadian clock and measuring the day length.
Interactions with other organisms
This was the most new and interesting piece of information that I found while researching this post. The ratio of red to far-red light actually affects how plants interact with herbivores, pathogens, and beneficial microorganisms (Ballaré and Pierik 2017). Plants that are adapted to grow in bright light are actually more susceptible to diseases and herbivores when they are being shaded, due to the regulation of the hormones salicylic acid and jasmonic acid by phytochrome. While this may seem like a bad strategy on the surface, the adaptive reason for it is that by allocating less energy to defense, the plant can allocate more energy to growth towards the canopy. Plants can even turn off their defense responses specifically in leaves and stems that are being shaded, in a “self-pruning” strategy; imagine a bunny coming along and discovering that the leaves growing in the shade aren’t as bitter as the leaves growing in the sun. If you are gathering herbs for food or medicine, it’s probably worth considering that the chemical properties of the plant will differ depending on the amount of sun it is receiving, and it wouldn’t surprise me if herbalists have known this for thousands of years.
Along the same theme, plants that are trying to escape shade have fewer interactions with beneficial bacteria and fungi, presumably because they can’t afford to feed those symbionts (Ballaré and Pierik 2017). There is also some evidence that when plants turn down their herbivory defences in the shade, they compensate by releasing chemicals to attract predatory insects to defend them from the insects that may eat them. Symbiosis is another scientific/spiritual passion of mine, so look for a post on that in the future!